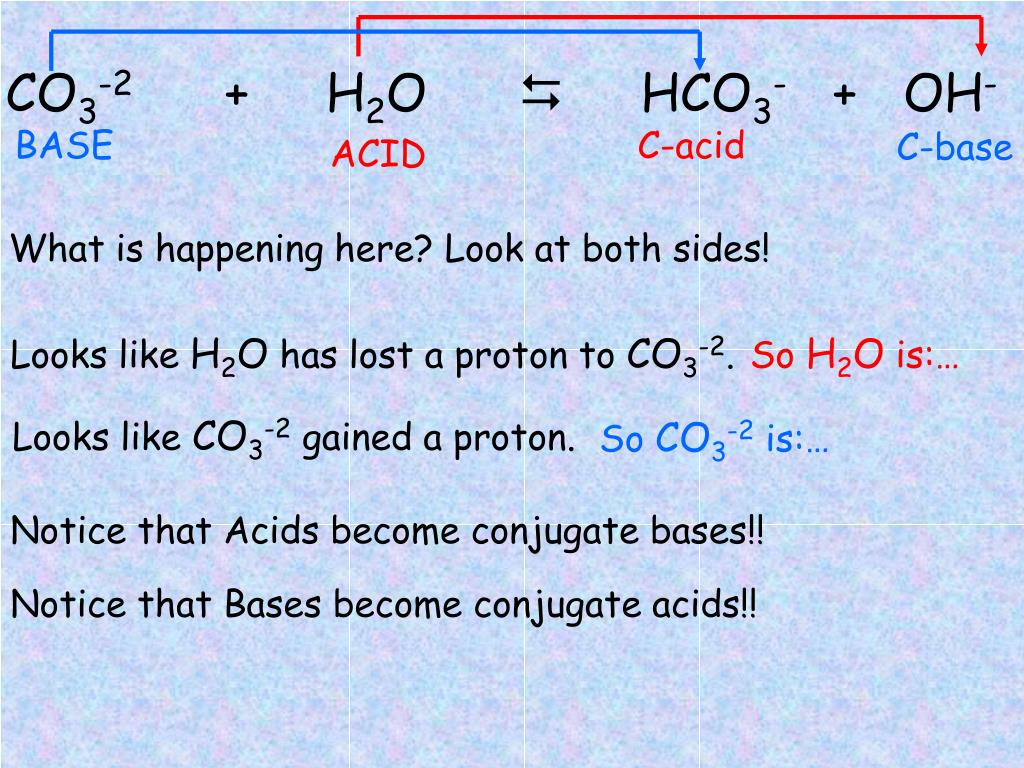
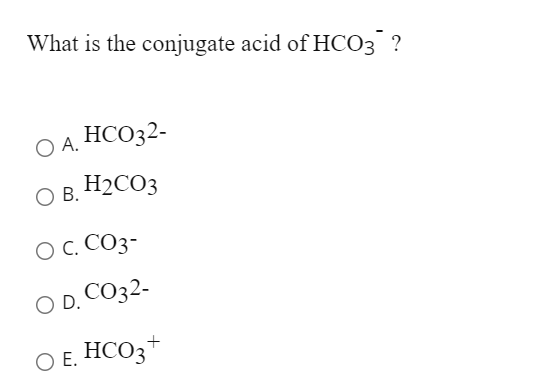Chemistry Help: The carbonate ion (CO3 2-) acts as a Bronsted base with water and equations - YouTube
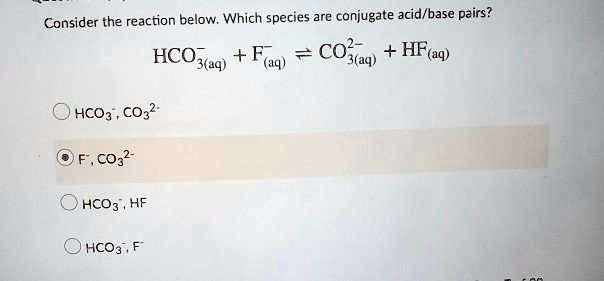
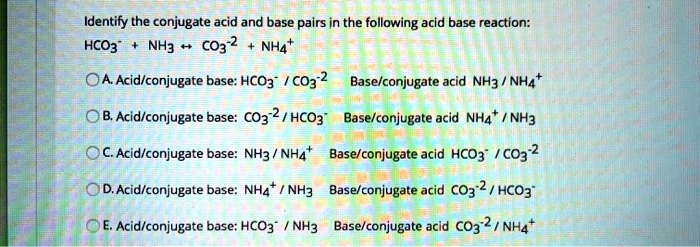
SOLVED: Consider the reaction below: Which species are conjugate acid/base pairs? HCO3(aq) + F(aq) CO3,aq) HF(aq) HCO3 , CO32* F , CO32- HCO3 HCO3 , F
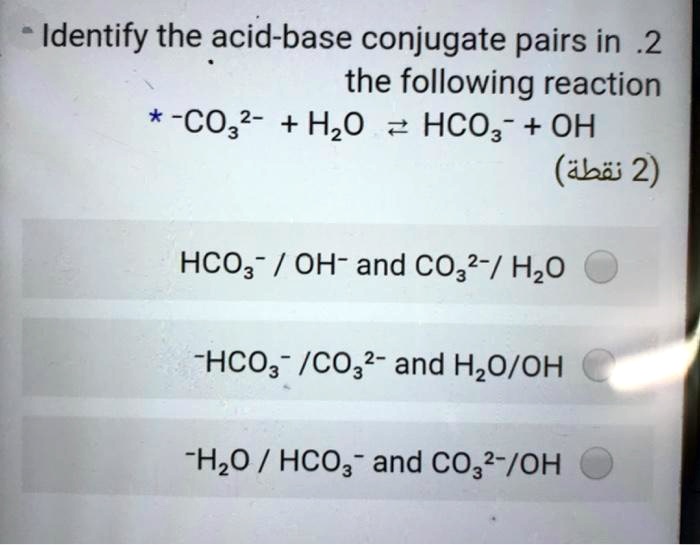
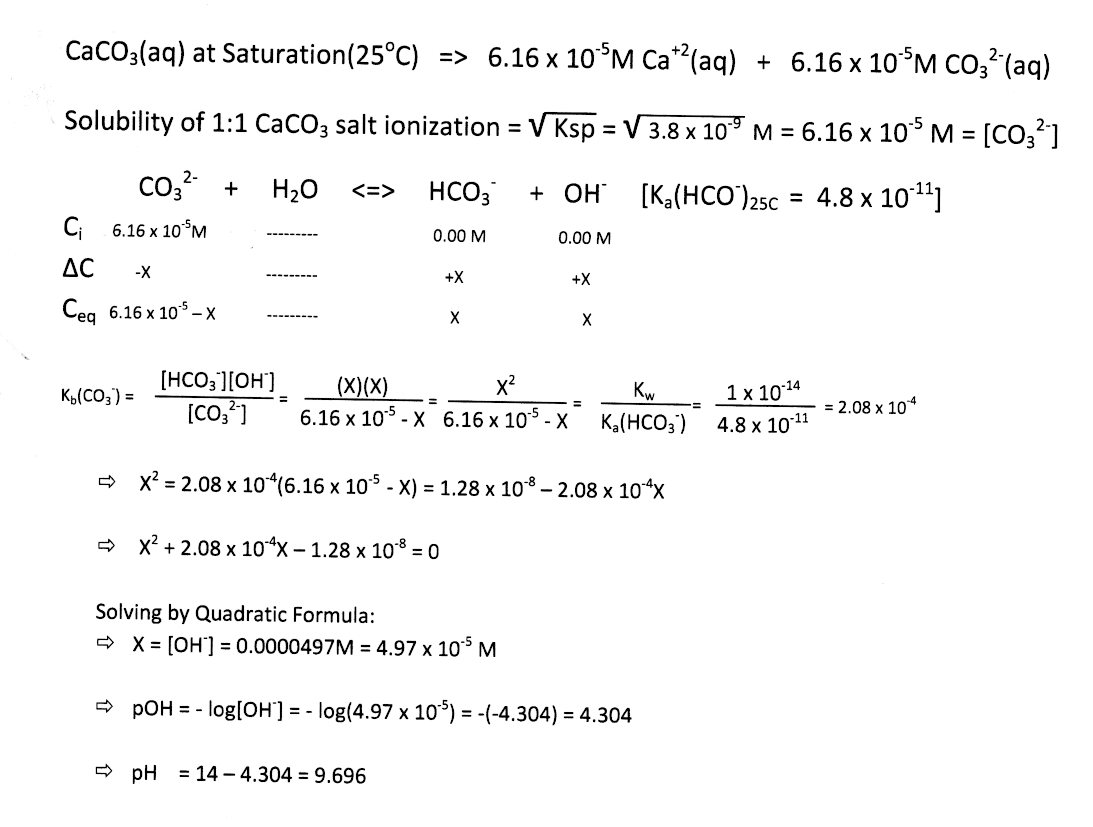
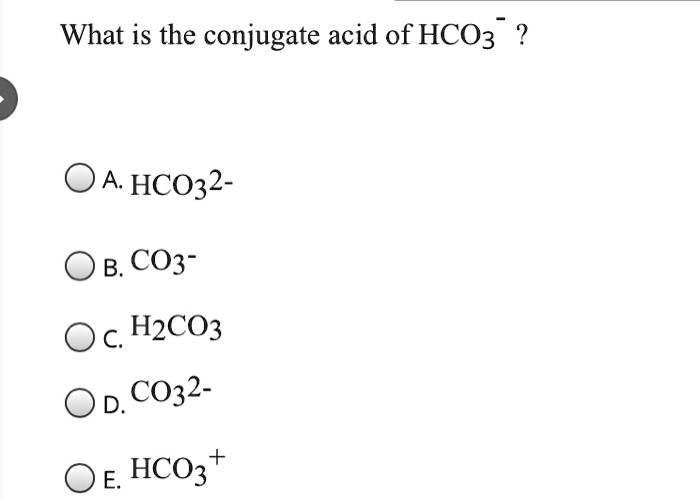
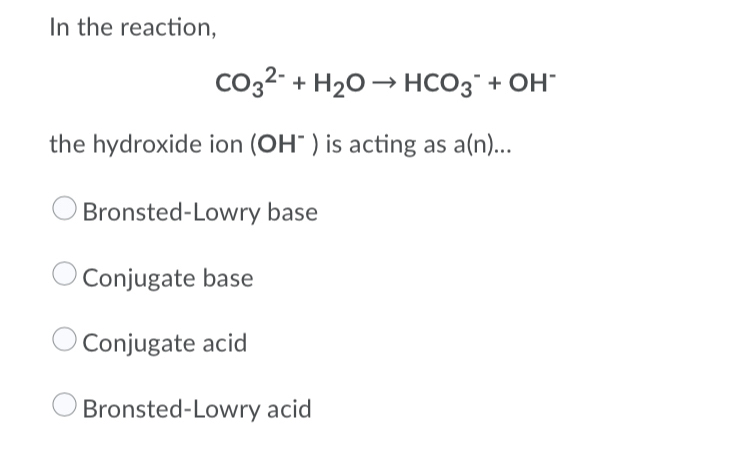
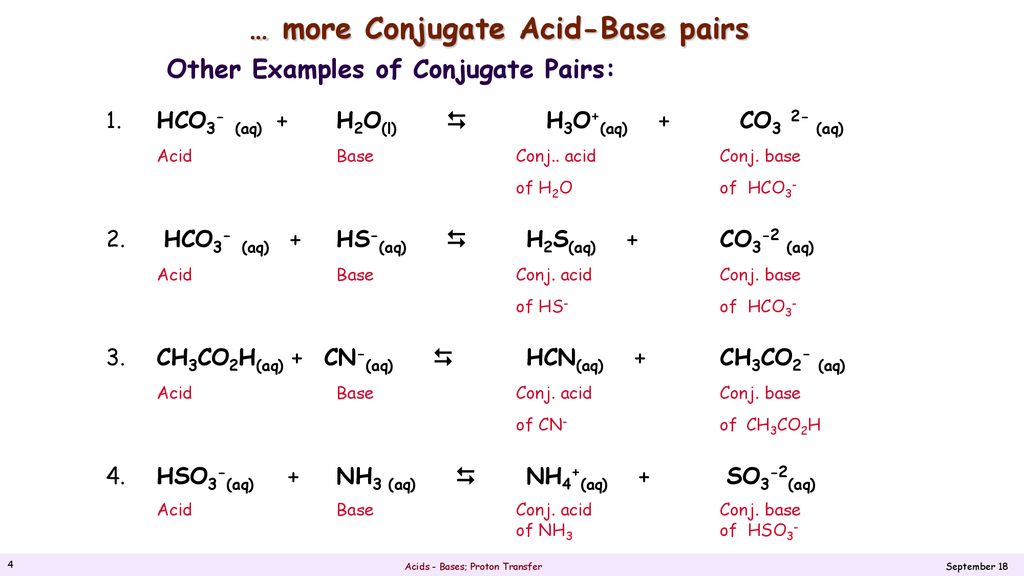
SOLVED: Identify the acid-base conjugate pairs in 2 the following reaction *-CO3 2 - +Hzo 2 HCO; +OH (abii 2) HCO3 1 OH- and CO3?-/ HzO HCO; - /CO32 2 - and HzO/OH Hzo / HCO: and CO3?-/OH